Executive Summary
WE BELIEVE IN COORDINATED ESSENTIALS FOR ADVANCING DNA / RNA VACCINES AND THERAPIES
Delivery technology
Formulation technology
Responsiveness and scale
Pivotal Partnerships

Orlance MACH-1™: Easy, Intuitive ‘Air Gun’ Vaccine Delivery

MACH-1 Maximizes Device Platform
Opportunities for DNA and RNA Vaccines
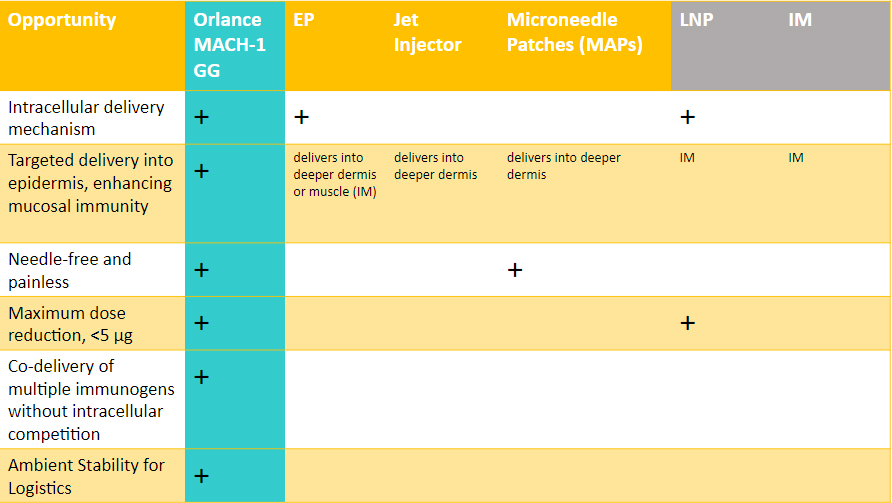
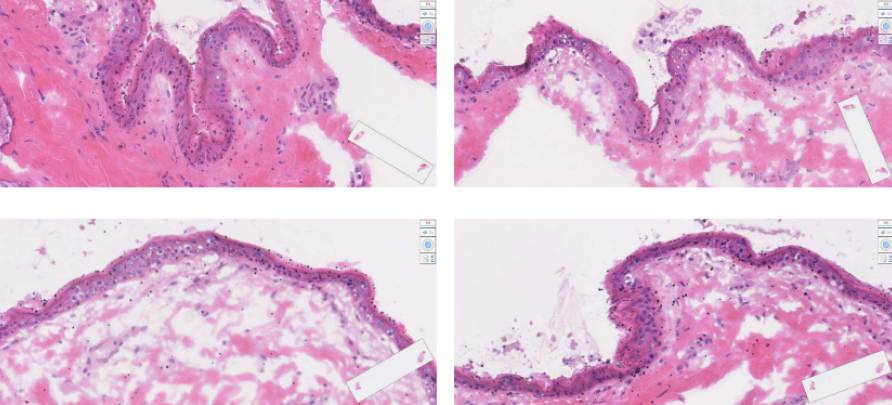
MACH-1 Uniquely Localizes Dose into Highly Immunogenic Epidermis
Orlance MACH-1 Pipeline
Orlance, Inc. Company Profile
Contact Information
Orlance, Inc.
1124 Columbia St., Suite 300
Seattle, WA 98104
206-792-5510
CEO
Kristyn Aalto
kaalto@orlance.com
Lead Asset Status
Kristyn Aalto
kaalto@orlance.com
Funding
$9.6M Non-Dilutive
(Plus $3M Non-Dilutive Pending)
PreSeed
Seed Raise 2023
Founded
2016
Founders
Kristyn Aalto, CEO, Director
Deborah Fuller, PhD, CTO, Director
James Mullins, PhD, Founder
Partnering Status
Early Partnering, No Current Field Restrictions, Seeking Co-Development
